The ovaries are the organs reesponsible for producing the female gametes (oocytes) and the sex hormones control the reproductive system organs and influence other organs in the body.
During a woman’s reproductive stage, a certain number of follicles (the combination of the oocyte and the cells containing it) is produced. One of these will be ovulated while the others will disappear as the result of cell death by atresia. Consequently, only some 400 to 500 oocytes of the 2,000,000 a woman has will reach ovulation during her lifetime.
The total number of follicles that a woman has at a given time is her ovarian reserve and determines the status of her fertility.
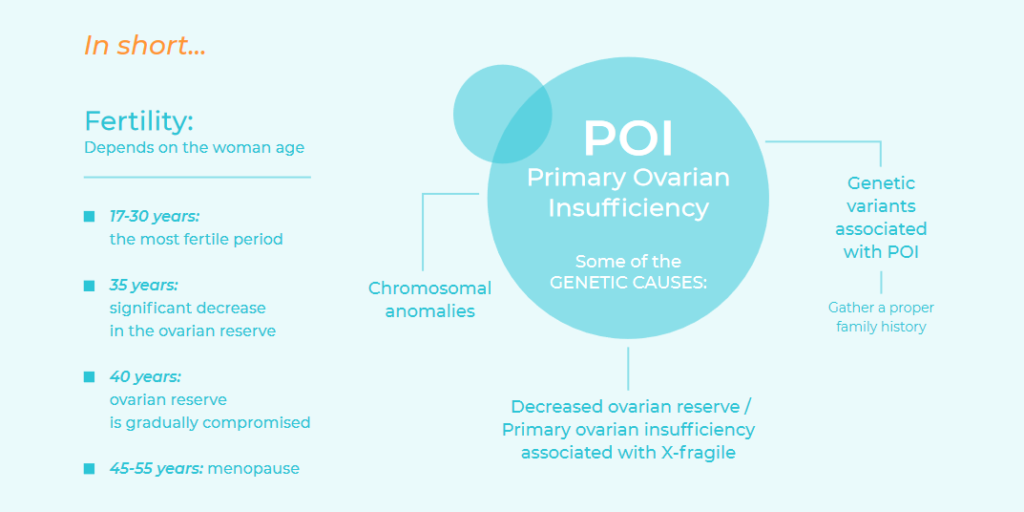
This depends mainly on how old the woman is, with the most fertile period being between the ages of 17 and 30. From the age of 35, there is a significant decrease in the ovarian reserve and, from the age of 40, the ovarian reserve is gradually compromised until it is completely exhausted, sometime between 45 and 55 years of age, when menopause usually begins.
In some cases, the decrease in the ovarian reserve occurs before it is expected. This is know as primary ovarian insufficiency (POI), which is characterised by the loss of ovarian function before the age of 40 or by a primary ovarian defect (primary amenorrhea). It is currently one of the main causes of female infertility. The fact that most women are currently planning their first pregnancy after the age of 30 is increasing the relevance of POI, whose prevalence is 1% before the age of 40 and 0.1% before the age of 30.
Since fertility begins to decrease about 20 years before menopause, and because when ovarian insufficiency becomes clinical and biochemically identifiable, the ovarian reserve is already severely reduced, there is justification for considering studies that allow us to predict a woman’s risk of premature menopause in order to consider bringing forward the age at which she gets pregnant or preserve the oocytes by freezing them.
The age at which a woman begins menopause is inheritable, and POI has a strong genetic component, in addition to other possible aetiologies such as autoimmune, metabolic, infectious or iatrogenic factors but, in most cases, it is classified as idiopathic. Epidemiological studies suggest an incidence of familial POI of 13% to 30%, showing that one-third of idiopathic POI is actually inherited A proper family history will make it possible to identify familial POI. This is of great importance since the risk of early menopause in direct female members who are female must be considered initially high in familial cases.
Some of the genetic causes of Primary Ovarian Insufficiency are:
- Chromosomal anomalies: The X chromosome has an essential role in maintaining ovarian function. Women with X monosomy – or Turner syndrome – have ovarian dysgenesis due to accelerated follicular atresia which typically results in primary amenrrhea. Turner syndrome has a prevalence of 1 in 2,500 girls born. In addition to X monosomy (50% of the patients with Turner syndrome), it may be produced by mosaicism (40% to 45%) and/or an anomalous chromosome. When the X monosomy is present with mosaicism, patients tends to have a less severe phenotype. Some 12% to 40% of 45X/46XX and 45X/47XXX mosaicisms have menstruations for several years until ovarian failure occurs.
X trisomy is also associated with ovarian dysfunction. This is the most common chromosomal anomaly in women, affecting 1 in 1,000 girls born. However, since most patients have mild or asymptomatic involvement, it is estimated that only 10% of cases with X trisomy are diagnosed.
Some X chromosome deletions and balanced translocations between an X chromosome and an autosome also cause POI. All these alterations are diagnosed by the peripheral blood karyotype study.. This should be requested for all women with POI.
- Decreased ovarian reserve and primary ovarian insufficiency associated with X-fragile: The FMR1 (Fragile X Mental Retardation type 1) gene is located on the X chromosome and contains a sequence of three nucleotides (CGG) that repeats f rom 6 to 44 times. When this number of repeats increases to 55 to 200 repeats, it’s called premutation and becomes unstable when transmitted to the offspring; it may increased to more than 200 repeats. The result of the complete mutation (>200 repeats) the complete silencing of the gene that causes Fragile X syndrome, the most frequent form of inherited intellectual disability in men. Of every 150 to 300 women, one is a carrier of a premutation in the FMR1 gene. Women who are carriers of a premutation may develop primary ovarian insufficiency or a decrease in the ovarian reserve.
Approximately 20% of women with a premutation will develop POI. Of women with spontaneous idiopathic POI, 2% to 6% will have a premutation on FMR1, while 14% of women with familial POI will have one. Therefore, the premutation on the FMR1 gene is the main known inheritable cause of both sporadic* and milial POI. Approximately 3% percent of women carrying the premutation will have irregular menstrual cycles during adolescence and altered hormonal profiles. Women carrying a premutation have a 5% to 10% risk of having a child with Fragile X syndrome. Consequently, the American College of Medical genetics (ACMG) and the American College of Obstetricians and Gynecologists* recommend carrying out a study of the FMR1 gene premutation to all women with POI or with a family history of it.
- Genetic variants associated with POI: Most often, POI has a highly variable expression in members of the same family, suggesting that it should be considered a multifactorial disease, which makes it extremely difficult to study. Although a large number of genes associated with POI have been published, it has not been possible to demonstrate causality in all cases and they are not accepted as diagnostic markers.
Therefore, it is important to gather a proper family history for each patient in order to preempt an irreversible situation of ovarian insufficiency and allow her to plan her pregnancy. A karyotype study should be requested for all patients with POI and a Fragile-X study for patients with POI and a normal karyotype, and all patients with a family history of POI.